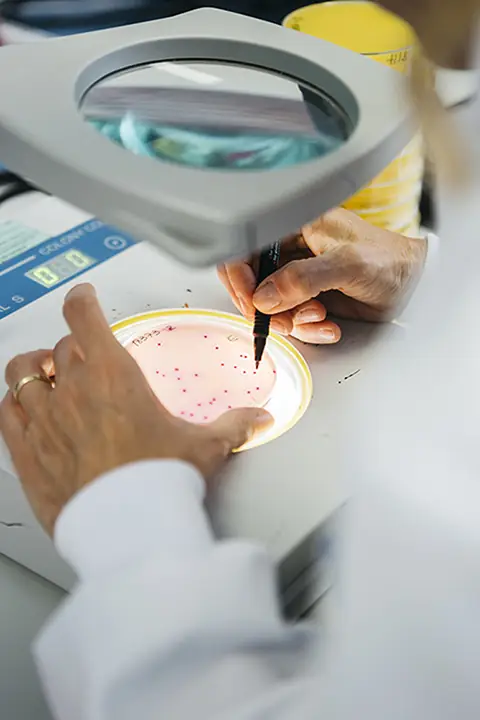
This validates the safety and stability of the product throughout its shelf life.
According to Regulation EC 1441/2007 (amendment of Regulation (EC) 2073/2005) on microbiological criteria for foods, agri-food companies must conduct, when necessary (especially in ready-to-eat foods), studies on compliance with the microbiological criteria throughout the product’s shelf-life to ensure its safety and stability.
The Listeria monocytogenes Growth Potential Assessment studies are conducted by Challenge test according to the guidelines in the document by the Community Reference Laboratory (CRL): "Technical Guidance: Shelf-life Studies for Listeria monocytogenes in Ready-to-eat foods".